Taking repeated blood samples from the same mouse is desirable as it allows a significant reduction in the number of mice used over the course of an experiment. It also means that an individual mouse can be its own control—having blood samples taken before and after a particular procedure—which scientifically may be the best way to do a particular experiment.
In the UK, the Home Office guidelines say that the maximum volume that can be taken from a mouse is <10% of total blood volume (TBV) on any single occasion to avoid hypovolaemic shock.
A similar rule applies to blood donation for humans where typically about 450 ml are collected and limited to <15% of TBV.
In laboratory animals, for repeated blood samples, this is typically limited to < 15% TBV in 28 days.
On average, mice have around 58.5 ml of blood per kg of bodyweight. So for an adult 30 g mouse TBV=1.75 ml and the maximum blood sample you can take is 175 μl. This is plenty for the biochemical methods used for analysing blood samples which require only small blood volumes. However, smaller blood volumes (20-40 μl) are required to be taken if repeated samples are needed or when using juvenile mice, which may weigh only 10-15g.
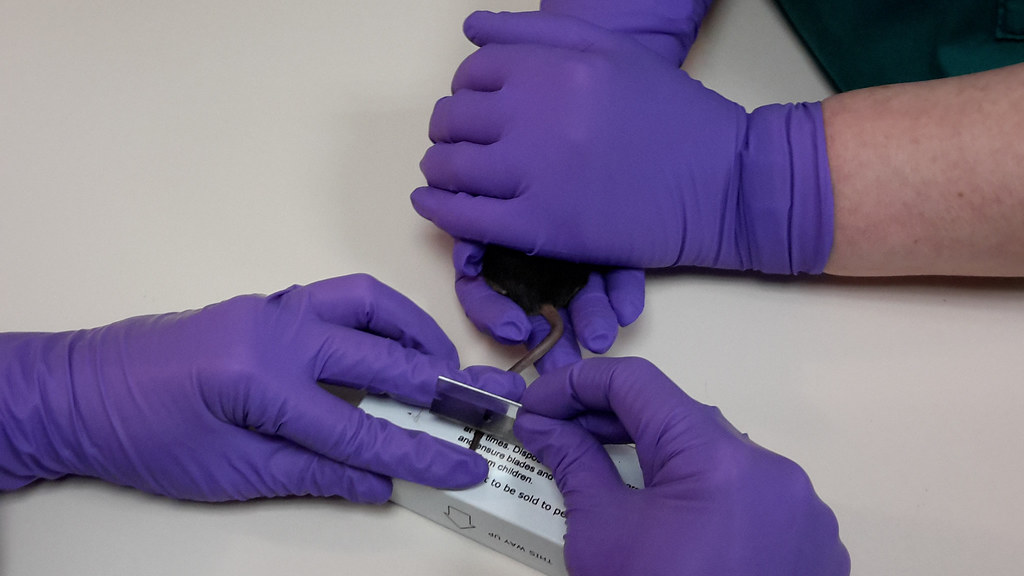
The tail incision method
In our research we are interested in studying the effects of stress, over time, on stress hormone responses and the development of depression related behaviours in mice. We have developed a refined minimally invasive method of blood sampling, which allows multiple samples to be taken from the same animal with as little stress as possible.
The tail incision method allows a small volume of blood, just enough for biochemical analysis, to be collected—a little like a pin prick in a human.
For this method the mouse is gently cupped by one person while the experimenter makes a small incision, about 2 cm from the tip of the tail, in the lateral tail vein using a sharp blade. Blood is directly collected into small volume capillary tubes. Blood flow starts and stops spontaneously through the small incision or with a small amount of pressure so no blood is “wasted".
This is a minimally invasive procedure with refined handling of the animals:
- no restraint devices were used
- no anaesthesia was used
- the smallest blood volume necessary was taken (and none wasted in needles or syringes)
- no tail warming was required
The procedure was demonstrated to be as stress-free as possible.
Additionally, the ability to take repeated samples from the same mouse at multiple time points reduces the number of animals required for an experiment. Thus, this method is in keeping with the 3R’s principles of both refinement and reduction of animal use.
Sadler, A. M. and Bailey, S. J., 2013. Validation of a refined technique for taking repeated blood samples from juvenile and adult mice. Laboratory Animals, 47 (4), pp. 316-319.