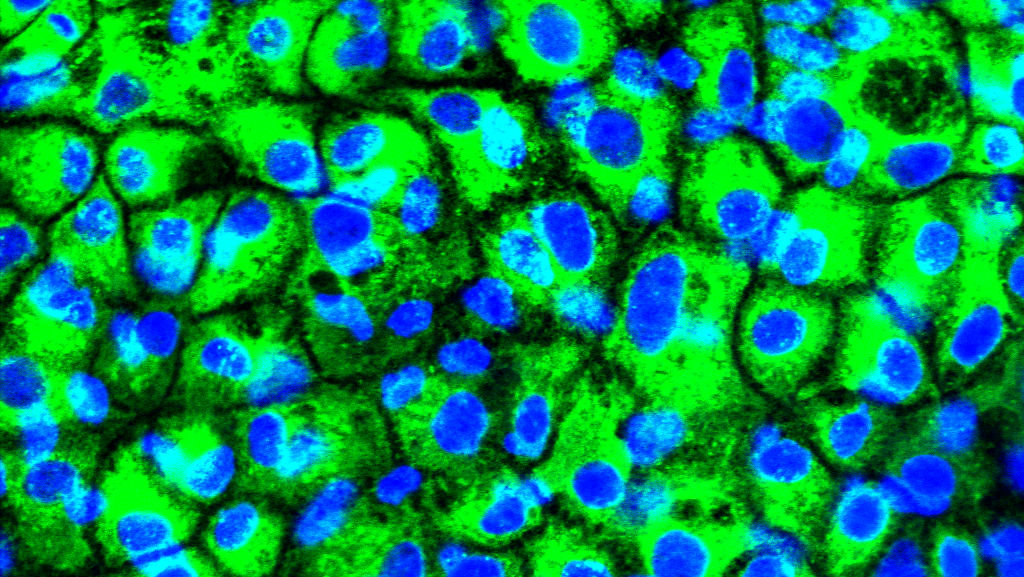
Stem cells are able to develop into more specialised cells and scientists believe they have huge potential to treat diseases or injuries that don’t currently have a cure.
The current method for developing precursor liver cells involves many different steps and uses a variety of biological agents.
The new process is much simpler, relying on just one small molecule, called 1M, being added to stem cell cultures. This treatment causes the stem cells to turn into precursors of liver cells.
Professor Melanie Welham and Dr David Tosh co-supervise the research. Professor Welham said: “The new method we have defined through our research is much simpler than previous procedures, so should reduce the cost of turning stem cells into precursor liver cells.
“This will allow the scale on which precursor liver cells are created to be more easily increased.”
Scientists are keen to develop liver cells that can be used to test the safety of new medical drugs. Even everyday painkillers are known to have toxic effects on the liver if taken in the wrong quantity, and the tests currently used don’t always accurately predict what will happen in humans.
Therefore, an improved supply of liver cells that can be used in testing the safety of new medicines will allow pharmaceutical companies to improve and strengthen the testing of drugs for human use.
Dr Tosh said: “This is a significant breakthrough in the field of stem cell research and will impact on the pharmaceutical industry and the way in which medicines are tested.
“There is, however, a great deal of work to still be done. Until now our research has focused on the early stages of liver cell development and there is still a lot of research to do to expand what has been found so far and to generate fully functioning liver cells.”
The research team has received funding from a public-private partnership called ‘Stem Cells for Safer Medicines’, a consortium of public funding bodies including the Medical Research Council, the Biotechnology and Biological Sciences Research Council, the Technology Strategy Board and the Department of Health, along with four pharmaceutical companies GlaxoSmithKline, AstraZeneca, Roche Healthcare and UCB Celltech.
The funding supports a five-year research programme, currently just starting its third year. Moving into phase two of the project the research team will now focus on improving the systems in the next stage of liver cell production.
The funding has supported the work of post doctoral researcher Dr Heather Bone, who has been instrumental in establishing the initial phases of this project, and has allowed Professor Welham and Dr Tosh to create two new research posts to work with Heather in phase two.
See the full paper in the Journal of Cell Science - 'Let there be liver'.
.............................................................................................................................................................................................
If you enjoyed this article you might also like:
New clues to how humble painkiller stifles cancer growth, May 2011
Scientists unveil 'switch' that could help treat autoimmune diseases, May 2011
New stem cell research could make lab mice redundant, Aug 2009