A project to measure the effects of electrical stimulation of a major nerve that could hold the key to new treatments for a range of diseases from epilepsy to depression is underway at the University of Bath.
Members of C3Bio, the University’s Centre for Biosensors, Bioelectronics and Biodevices, are working to improve understanding how the body reacts to electrical stimulation of the vagus nerve. Knowing more about how the body responds to this stimulation - known as neuromodulation - will help to develop future therapies.
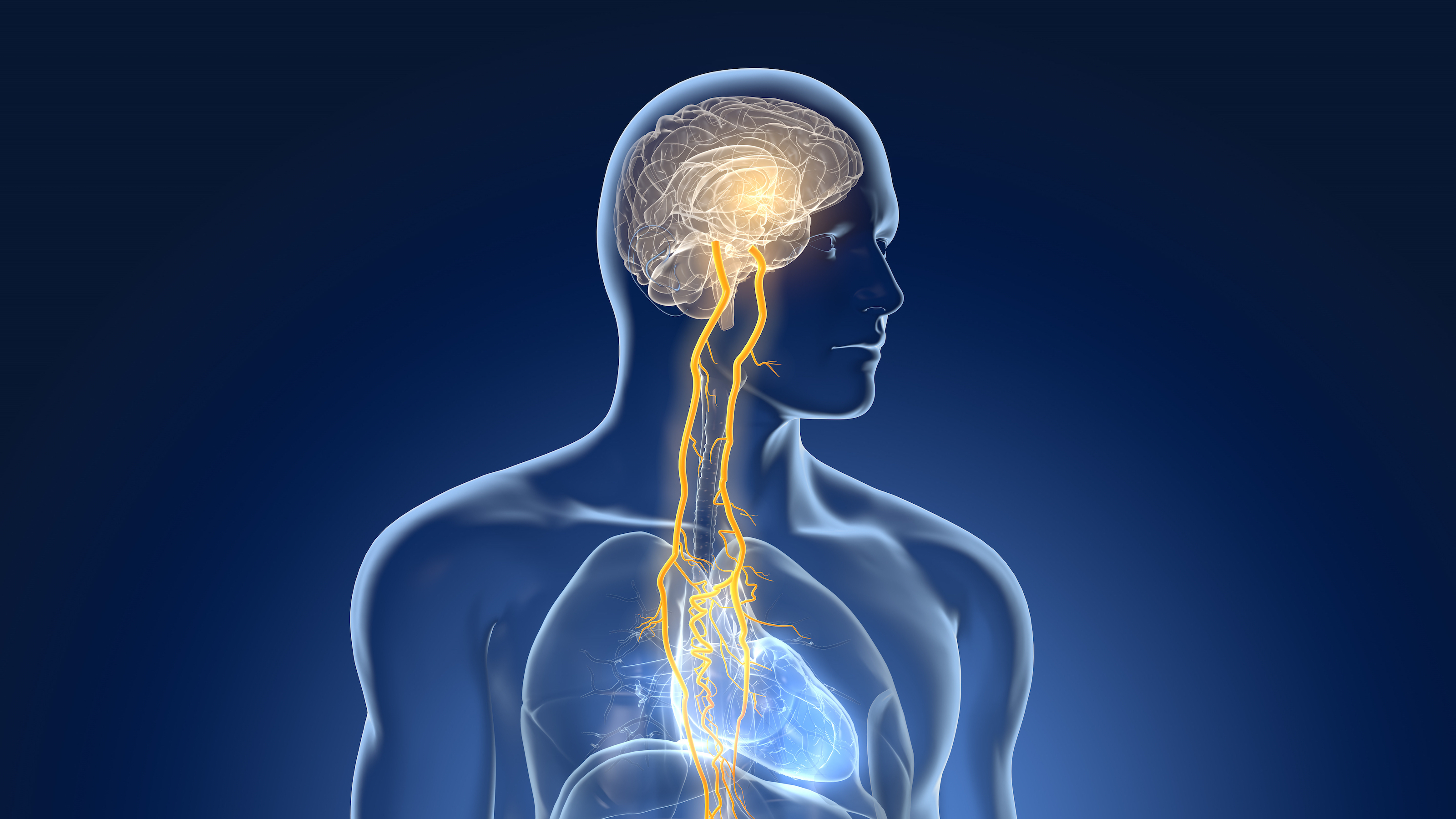
The vagus nerve, actually a bundle of nerves which connects the brain to the heart, lungs and digestive system, has the potential to unlock new therapies for a wide range of diseases including epilepsy, depression, arthritis and asthma via electrical stimulation.
The project aims to understand exactly how to isolate and measure the effect of electrical stimulation on the nerve and its peripheral systems, something existing treatments and devices lack. Recording these effects will help to develop and optimise treatments, enable personalised care, achieve greater clinical efficacy, and minimise any side effects.
Dr Ben Metcalfe, a lecturer in Electronic & Electrical Engineering and lead member of the project, says: “The vagus nerve has huge potential for electrical stimulation treatments as it connects the brain with many parts of the body. It could be used to treat a startling number of diverse disorders ranging from rheumatoid arthritis to epilepsy, asthma and depression.
“One of the challenges presented by taking this approach is really understanding the direct effect of stimulation. A fundamental limitation of current approaches is that we don’t know the direct effect of the stimulation - we have to wait until some physiological effect is observable. This can take time, so we can only draw partial conclusions from current treatments.
“This project will develop a way of stimulating the nerve and ‘listening’ to resulting activity through the nervous system in real time, and cutting out the ‘noise’ of other bodily functions. So we’ll have a much better understanding of how to develop and fine-tune smart and reactive therapies, even for individual patients.”
Recording this activity is carried out with an ENG – an electroneurogram. This monitors signals within a nerve by attaching directly to it – similarly to how an electrocardiogram attaches to and monitors the heart.
C3Bio is partnering with technology company TTP (The Technology Partnership) to further the understanding of the effect of neuromodulation with a view to creating new therapeutic devices. The partnership will initially focus on epilepsy treatments, as products to alleviate symptoms of the condition are already established on the market and accepted by clinicians and patients.
Dr Metcalfe adds: “Our partnership with TTP has been instrumental in enabling us to start the translation of our research. TTP is able to provide us with both commercial relevance, as well as expertise in device development and modelling, that are helping us bridge the gap between academia and industry.”
Dr Chris Dawson, who leads the Neurotechnology Team at TTP, says: “The work that C3Bio are doing is advancing our understanding of neuromodulation and its interaction with the nervous system and promises to revolutionise the treatment of a wide range of diseases. Sensing very small nerve signals using techniques like velocity selective recording (VSR) are enabling us to respond to stimuli in real time, and the ability to implement these in low-power, small-form-factor implantable devices is enabling the next generation of neuromodulation technologies.”