In a world of sedentary jobs and hyper-processed diets, it’s unsurprising that obesity levels and their associated risks are on the rise. In the last 30 years alone, worldwide adult obesity has more than doubled, with an estimated 1 in 8 people living with obesity. With it has come a spike in serious health consequences, like type 2 diabetes and cardiovascular disease.
But developmental geneticist, Dr Kim Moorwood, and her team believe there’s more to the story than meets the eye.
Studies have long shown a connection between early development and later-life health. Babies that are born small, for example, are more likely to die from cardiovascular disease - regardless of their lifestyle in adulthood.
For the last twenty years, Kim has been working alongside Professor Andrew Ward at the University of Bath to try and understand why.
Searching for genetic clues
If we can understand what links fetal growth and later-life health, Kim hopes early interventions can reduce the risk of common health conditions in adulthood. Much like folic acid supplements in pregnancy reduce the risk of spina bifida, simple interventions could encourage babies to put on more, or less, fat at crucial stages of development, altering their chances of later-life disease.
Using mice as a model, the team are exploring how genes and environment interact before and soon after birth; and how these influence levels of body fat and health in later life. Mice are a particularly good organism to use because their entire genome is known. Examining mouse strains with specific genes deleted allows researchers to discover which genes control a whole host of physiological processes, from fetal growth to fat deposition in mammals.
Despite the differences between mice and people, the basic genetic, metabolic and physiological processes involved in growth and development occur in much the same way. A natural lifespan of around 2-3.5 years means changes can also be tracked throughout life more readily than in human populations.
The team measured the fat/lean body composition of offspring from weaning, and then at 6-weekly intervals throughout their lives. They also monitored individuals’ insulin response at multiple life stages. Repeated sampling over time produced strong data, while minimizing the number of animals needed.
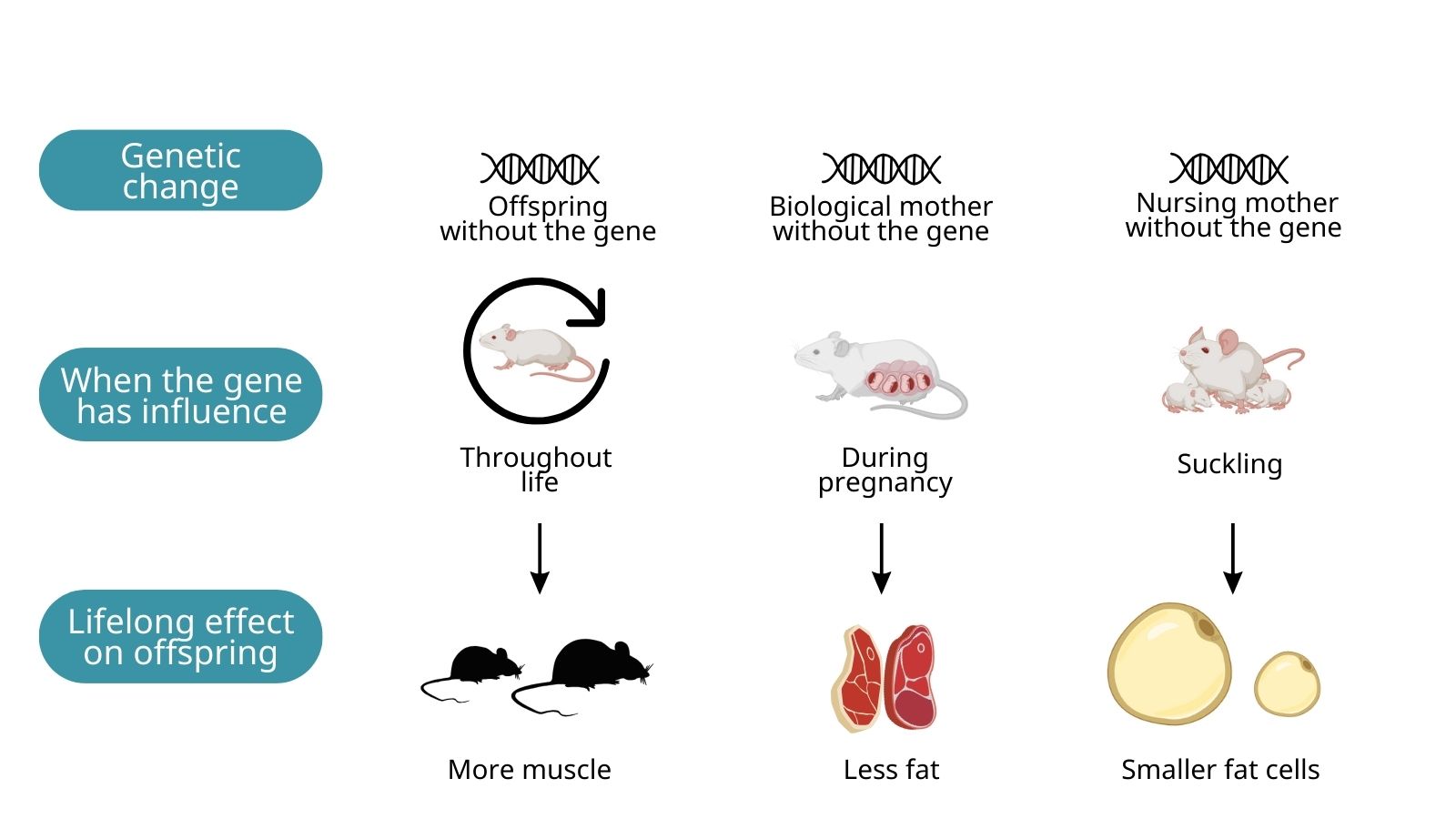
As well as comparing individuals with and without a specific gene, they also studied the effect of deleting the gene in the mother. This revealed that the mother's gene could influence the pup during pregnancy or suckling, altering a pup's fat levels in later life.
Finally, the team examined the role of the developmental environment by comparing different diets during pregnancy. Mathematical modelling then reveals lifelong trends between the groups at different life stages.
'It’s amazing to see the power of a single gene in the mother affecting both fat levels and insulin response throughout their offspring’s life,' said Kim.
'This effect was stronger and opposite to the effect of poor maternal diet in altering fatness. We were surprised to find that while mothers lacking the gene produced offspring that accumulated less fat, these same offspring had a slightly impaired insulin response - the opposite of what you might expect. So the story is not going to be a simple one.'
Introducing Grb10
The research carried out by Andrew Ward’s group has focussed on a gene called Growth factor Receptor Bound protein 10, Grb10 for short.
Earlier studies of mice showed that Grb10 restrains fetal growth, regulating the amount of nutrients that pass from mother to young and preventing babies from developing too fast. The gene is also known to regulate insulin signalling, dampening the body’s response to insulin, something that in later life can be a precursor to type 2 diabetes.
Without this gene controlling growth, mouse pups lacking Grb10 were born heavier and larger than those with the gene. As adults, mice lacking the gene were leaner and more sensitive to insulin. While humans also have the Grb10 gene, it’s not yet known how strong the parallels between mouse and human responses are.
Due to the gene’s dual effects, impacting both fetal growth and adult metabolism, Kim dug further into its role, this time turning her attention to the mothers.
The maternal touch
In humans, as in all mammals, the rate of growth of young during early life is dependent upon the mother. Maternal nutrition and other factors during pregnancy can influence the growth rate of young in early life, but how this links to later life health is not yet known.
To see if there were any distinct effects of Grb10 absence in either pregnant or suckling mothers, newborn litters were switched from one mother to another at birth. During their study, the team discovered that mice nursed by a mother lacking the Grb10 gene were less fat than normal during suckling. In later life, those same young had smaller fat cells in adulthood.
Surprisingly, mouse pups born to biological mothers lacking the gene, but who were nursed by normal mothers, were also found to have around a third less fat, long into adulthood. Together, these findings suggest that fat levels throughout life are, to some extent, programmed before birth and in early life by a process involving the Grb10 gene.
'We expect similar mechanisms to work in people,' said Kim, 'but our mouse experiments have enabled us to show cause and effect. This would be much harder to show in people.'
'Studies have begun using modern technology to measure fatness rather than just birthweight in newborn babies, but we will have to wait a lifetime to track their lifelong health. In mice, we now have a key to unlock other parts of the process of programming lifelong fat levels, which will lead to a fuller understanding of how nature and nurture are intertwined.'
Taking the next steps
In the future, the team hope to secure funding to follow up on this lead. Further studies would explore the role the gene plays in the mother that alters later-life fat levels in her offspring, including when and where the function is found.
By understanding the developmental origins of different fat deposits in the body, and how processes before birth can influence them, researchers could uncover opportunities for tackling obesity in future populations. This could even be through simple interventions, such as dietary advice for pregnant women.
However, as mammals have evolved, so too have the processes behind our development - and these processes likely have important benefits. An increased chance of survival at birth for example could come at the expense of later-life health. Before translating any links to human therapies, we must fully understand the mechanisms at work and the potential cost of making changes.